Abstract
Purpose
We report results of the first German prospective multicenter single-arm phase II trial (ARO 2013-06; NCT02635256) of hypofractionated robotic stereotactic body radiotherapy (SBRT) for patients with localized prostate cancer (HYPOSTAT).
Methods
Patients eligible for the HYPOSTAT study had localized prostate cancer (cT1‑3 cN0 cM0), Gleason score ≤ 7, prostate-specific antigen (PSA) ≤ 15 ng/ml, prostate volume ≤ 80 cm3, and an International Prostate Symptom Score (IPSS) ≤ 12. Initially, inclusion was limited to patients ≥ 75 years or patients 70–74 years with additional risk factors. The trial protocol was later amended to allow for enrolment of patients aged ≥ 60 years. The treatment consisted of 35 Gy delivered in 5 fractions to the prostate and for intermediate- or high-risk patients, also to the proximal seminal vesicles using the CyberKnife system (Accuray Inc., Sunnyvale, CA, USA). Primary endpoint was the rate of treatment-related gastrointestinal or genitourinary grade ≥ 2 toxicity based on the RTOG scale 12–15 months after treatment. Secondary endpoints were acute toxicity, late toxicity, urinary function, quality of life, and PSA response.
Results
From July 2016 through December 2018, 85 eligible patients were enrolled and received treatment, of whom 83 could be evaluated regarding the primary endpoint. Patients mostly had intermediate-risk disease with a median PSA value of 7.97 ng/ml and Gleason score of 7a and 7b in 43.5% and 25.9% of patients, respectively. At the final follow-up 12–15 months after treatment, no patient suffered from treatment-related gastrointestinal or genitourinary grade ≥ 2 toxicity. Acute toxicity was mostly mild, with three grade 3 events, and the cumulative rate of grade ≥ 2 genitourinary toxicity was 8.4% (95% CI 4.1–16.4%). There were no major changes in urinary function or quality of life. The median PSA value dropped to 1.18 ng/ml 12–15 months after treatment. There was one patient who developed distant metastases.
Conclusion
Robotic SBRT with 35 Gy in 5 fractions was associated with a favorable short-term toxicity profile. Recruitment for the HYPOSTAT‑2 trial (ARO-2018‑4; NCT03795337), which further analyses the late toxicity of this regimen with a planned sample size of 500 patients, is ongoing.
Similar content being viewed by others
Introduction
Radiation therapy is a standard treatment modality for patients with localized prostate cancer. The randomized controlled ProtecT trial showed equivalent overall survival for radiotherapy with short-term androgen deprivation therapy (ADT) compared to surgery for patients with localized prostate cancer [1]. Traditionally, radiotherapy has been applied using conventional fractionation with single fractions of 1.8–2 Gy in 5 fractions per week over the course of 7–8 weeks. However, preclinical and clinical studies have suggested an α/β value of 1.5–2 for prostate cancer [2], which is considerably lower than for many other tumors and even lower than the α/β value of surrounding organs such as the bladder and the rectum.
This provides a strong rationale for the use of hypofractionation in the treatment of prostate cancer. A multitude of randomized controlled trials have been conducted using moderate hypofractionation with single doses of 2.5–3.5 Gy [3]. Overall, moderate hypofractionation resulted in equivalent oncological outcomes. There were some signs of increased acute gastrointestinal (GI) and genitourinary (GU) toxicity, but chronic toxicity was similar. Moderate hypofractionation has been accepted as a standard of care for localized prostate cancer in almost all clinical situations [4].
Stereotactic body radiotherapy (SBRT) has been employed to treat extracranial tumors in bone, liver, and lung for over 20 years [5, 6]. Initial reports of SBRT for prostate cancer using ultra-hypofractionation with 5 fractions were published in 2009 [7]. In 2016, the German S3 guideline introduced SBRT for prostate cancer for the first time. The statement mandated the conduct of SBRT within the context of prospective clinical trials. Thus, the HYPOSTAT trial was designed [8]. We herein report the final results of the HYPOSTAT trial.
Materials and methods
HYPOSTAT was a multicenter prospective single-arm trial (ARO 2013-06; NCT02635256) investigating the use of robotic SBRT using the CyberKnife-System (Accuray Inc., Sunnyvale, California, USA) for patients with localized prostate cancer. The study protocol has been published previously [8]. The study was approved by the ethics committee of the University of Lübeck (leading ethics committee, file number 13–052) as well as the local ethics committees at the participating sites.
Eligibility criteria
The inclusion criteria were localized prostate cancer (i.e., no evidence of nodal or distant metastases), Gleason score ≤ 7, prostate-specific antigen (PSA) < 15 ng/ml, prostate volume < 80 cm3, IPSS ≤ 12, age > 75 years or age 70–75 years, and either PSA > 10 ng/ml and/or Gleason score 7b and/or Gleason score 7a with > 33% positive biopsy cores and/or cT > 2a and/or prostate volume > 60 cm3. Patients were not eligible in case of previous radiotherapy to the pelvis, contraindications against the implantation of fiducials, immunosuppressive therapy, relevant comorbidities interfering with the study procedures, or patient’s inability to understand or comply with the procedures.
At the time of study conception, the PREFERE trial, a large randomized controlled phase III trial comparing active surveillance, surgery, and different radiotherapy modalities, was recruiting in Germany [9]. If patients were eligible for the PREFERE-trial, enrolment into the PREFERE trial was favored. After the closure of the PREFERE trial in 2016, there was an amendment for the HYPOSTAT trial, allowing enrolment for patients aged ≥ 60 years without any additional age-based restrictions.
Treatment planning and administration
Patients received implantation of 3–4 fiducials into the prostate. The planning CT scan (slice thickness ≤ 1.5 mm) was acquired ≥ 5 days after fiducial implantation. A minimum bladder filling of 20–30 ml was intended, placement of a foley catheter for potentially better urethra delineation was optional, as previously described [8]. To achieve optimal bowel preparation, self-administration of a daily rectal enema was intended. A planning MRI for precise prostate and critical structure delineation was required and coregistered with the planning CT scan as per the recommendations for SBRT practice [5, 6]. The gross tumor volume (GTV) was defined as the prostate for patients with low-risk prostate cancer. For intermediate- and high-risk patients, the proximal seminal vesicles (intersection between the seminal vesicles and a 1-cm expansion of the prostate contour) were included in the GTV.
The clinical target volume (CTV) was generated by expanding the GTV by 1–2 mm. A 3-mm margin was added around the CTV (dorsally 1 mm) to generate the planning target volume (PTV). The prescription dose was 35 Gy in 5 fractions (PTV V35Gy ≥ 95%) to the PTV-encompassing 75–85% isodose (80–85% if the urethra was not contoured). The GTV should be covered by the 37.5 Gy or 38.5 Gy isodose for patients with low- (GTV V37.5 Gy ≥ 95%) or intermediate- to high-risk prostate cancer (GTV V38.5 Gy ≥ 95%), respectively. Organ at risk constraints and the urethral high-dose-sparing protocol have been published previously [8].
Treatment was performed using the CyberKnife system with translational and rotational tracking using the fiducial markers. Treatment was administered every other day until the post-PREFERE amendment and could be administered daily or every other day based on treatment center preference thereafter, with a maximum treatment duration of 2 weeks. As this amendment was designed, the decision was made to include a subgroup analysis according to the treatment schedule after discussion with the lead trial statistician (RV).
The use of ADT was allowed and discussed on an individual basis.
Endpoints and statistical hypothesis
The primary endpoint was the rate of treatment-related grade ≥ 2 genitourinary (GU) and gastrointestinal (GI) toxicity based on the Radiation Therapy Oncology Group (RTOG) scale 12–15 months after treatment. Secondary endpoints were acute toxicity using the Common Terminology Criteria for Adverse Events (CTCAE) scoring system (version 4.03), late toxicity using the RTOG scoring system, urinary function based on the International Prostate Symptom Score (IPSS), quality of life (QOL) using the European Organisation for Research and Treatment of Cancer (EORTC) QOL questionnaire C30 and the Patient Oriented Prostate Utility Scale (PORPUS) questionnaires, as well as PSA response after radiotherapy.
Study visits were scheduled at each treatment session (V02–06), and 4–6 weeks (FU-01), 3 months (FU-02), 6–9 months (FU-03), and 12–15 months (FU-04) after treatment, as described previously [8]. PORPUS was completed at V01, V06, and FU-01 to FU-04. EORTC QLQ-C30 was scheduled at baseline and FU-04. IPSS and PSA were determined at baseline and during FU-01 to FU-04. Biochemical progression was defined according to national prostate cancer guidelines. In case of biochemical progression, standard imaging (CT/MRI of the abdomen and pelvis and bone scan) with the addition of PSMA PET-CT in case of negative findings was performed.
Based a literature review [8], we assumed toxicity rates of 17.5% (GU) and 10% (GI) 1 year after standard treatment. Using meta-regression, we estimated grade ≥ 2 toxicity rates for SBRT of 2.8% for the GU tract and 1.1% for the GI tract at FU-04. Sample size calculation yielded a patient number of n = 85 patients with a statistical power of 78% and 88% and a two-sided significance level of 5% using the exact binomial test with a Bonferroni–Holm procedure to demonstrate superiority of SBRT. A planned subgroup analysis was conducted to assess the difference between daily vs. every-other-day treatment and reported toxicity.
Results
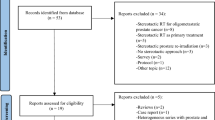
From July 2016 through December 2018, 88 patients were enrolled. After exclusion of screening failures (n = 3), 85 patients received treatment at three trial sites and formed the full analysis set (FAS), which was identical to the safety analysis set in this trial. Two patients discontinued the trial due to diagnosis of pancreatic cancer (end of treatment: V06) and glioblastoma (FU-01). Thus, 83 patients were available for primary endpoint analysis. All of these patients completed the trial-mandated follow-up of 12–15 months. Per-protocol analyses (PP) were conducted using data from 74 patients with exclusion of 7 patients who had follow-up visits outside the predefined timeframe. The Consolidated Standards of Reporting Trials (CONSORT) diagram is shown in Fig. 1.
Patient characteristics are listed in Table 1. Patients mostly had intermediate-risk disease with a median PSA value of 7.97 ng/ml and a Gleason score of 7a and 7b in 43.5% and 25.9% of patients, respectively. ADT was used in only 3 patients. The dose was prescribed to the 75–83% isodose line (mean: 80%) with 92.1–98.6% coverage (mean: 95.6%), and the PTV D98% and D2% and GTV D50% were calculated at 32.6–35.2 Gy (mean: 34.0 Gy), 42.2–46.7 Gy (mean: 43.9 Gy), and 39.1–42.6 Gy (mean: 40.1 Gy), respectively. Foley catheters were not used. No major protocol dosimetry violations for critical structures were observed.
Safety
Acute toxicity according to CTCAE-criteria was evaluated from V02 until FU-02. Overall, high-grade toxicity was rare and limited to one case each of grade 3 irritative symptoms, proctitis, and incontinence. The number of patients with the maximum grade of a toxicity according to CTCAE during the course of the study is shown in Table 2. For all adverse events reported, intensity was mostly mild, with 24 grade 2 events. The most common adverse events were irritative symptoms and proctitis.
Late toxicity was determined using the RTOG scoring system. In the FAS, there were two cases of grade 3 bladder toxicity (2.41%), corresponding to temporary incontinence that resolved during follow-up and 5 cases of temporary grade 2 bladder toxicity (6.02%). There was no grade 2–3 GI toxicity.
At the final follow-up 12–15 months after treatment (FU-04), no patient suffered from treatment-related GI or GU grade ≥ 2 toxicity (Bonferroni–Holm-adjusted Wilson Score 95% confidence interval [CI] 0–5.7% for the FAS and 0–6.4% for the PP data). Thus, the null hypotheses could be rejected. The cumulative rate of grade ≥ 2 GU toxicity was 8.4% (95% CI 4.1–16.4%) in the FAS and 9.5% (95% CI 4.7–18.3%) in the PP. Figure 2 shows the cumulative grade ≥ 2 GU toxicity.
Efficacy
One patient had biochemical disease recurrence and developed distant metastases during follow-up. There was no local recurrence, and the median PSA value dropped to 1.18 ng/ml at the last study follow-up 12–15 months after treatment. The course of PSA measurements during the study are shown in Fig. 3.
Quality of life and patient-reported outcomes
QOL assessment with the EORTC QLQ C30 questionnaire demonstrated no impairment of quality of life between baseline and end of study (Supplementary Table 1). Overall QOL remained stable. The median total score for FAS was 95.71 (95% CI 94.02–97.44) at baseline and 95.94 (95% CI 94.23–97.44) at FU-04.
PORPUS total scores showed little variation during the study. The median total score at baseline for FAS was 91.5 (95% CI 87.5–93.5) at baseline and 92.0 (95% CI 89.0–93.0) at FU-04. The median of difference compared to baseline was largest at V01 and FU-03 with +2 (95% CI 0–2 for V01 and 95% CI 0–4 for FU-03). The course of PORPUS total scores for FAS and PP is listed in Supplementary Table 2.
IPSS slightly increased from baseline. The median IPSS for FAS was 6.0 (95% CI 5–7) at baseline and 7.5 (95% CI 6–9) at FU-04, the median increase was 2 (95% CI 1–3). Results regarding IPSS for FAS and PP are shown in Supplementary Table 3.
Subgroup analysis of daily vs. every-other-day treatment
Results of the subgroup analysis regarding daily vs. every-other-day treatment are shown in Table 4. The majority of patients received their treatment every other day (50 patients, 60.2%). One trial center routinely used daily treatments (n = 33), while the other two trial centers preferentially treated patients every other day. There was a significantly higher incidence of CTCAE grade GI toxicity and proctitis as well as bladder toxicity according to the RTOG classification in the patients with daily treatment. Nocturia was more frequently documented in patients receiving their treatment every other day.
Discussion
Our results demonstrate the feasibility and tolerability of robotic SBRT with 35 Gy in 5 fractions for patients with localized prostate cancer.
Since conception of the HYPOSTAT trial, evidence for SBRT in the treatment of patients with prostate cancer has considerably improved. In 2019, a meta-analysis of 38 prospective single-arm clinical trials, case series, and registries with a total of 6116 patients was published [10]. Biochemical control was high, with a 5-year rate of 95.3% (95% CI, 91.3–97.5%). Acute GU and GI grade 2 toxicity occurred in 15.5% and 6.1%, respectively. Late grade 2 toxicity occurred in 12.1% and 4.9% of patients for the GU and GI tract, respectively. An increasing biologically effective dose was associated with increased biochemical control, but also with increased late grade ≥ 3 GU toxicity [10].
To date, two randomized controlled trials have reported results. The HYPO-RT-PC-trial randomized 1200 patients to definitive radiotherapy with 78 Gy in 39 fractions or to SBRT with 42.7 Gy in 7 fractions [11]. In this trial, 80% of patients were treated with 3D conformal radiotherapy and 90% of patients had fiducials implanted. Almost 90% of patients had intermediate-risk disease, as patients with low-risk disease could not be enrolled. Non-inferiority of SBRT in terms of failure-free survival was demonstrated. There was a slight but significant increase in acute GU and GI toxicity up to 1 year in the case of GU toxicity, but long-term toxicity was similar between the two arms. The PACE‑B trial compared SBRT with 5 × 7.25 Gy to conventional or moderate hypofractionation in a trial population of 874 patients with low- or intermediate-risk prostate cancer [12]. SBRT was delivered with robotic SBRT in 41% of patients and with conventional linear accelerators in 59% of patients. Only 73% of patients in the SBRT arm received fiducials. All patients in the standard arm received intensity-modulated radiotherapy. Acute toxicity results up to 12 weeks after the end of radiotherapy have been published [12]. Worst acute toxicity grade ≥ 2 according to RTOG was similar between the arms. Toxicity grading according to CTCAE showed significantly higher rates of grade ≥ 2 GU and GI acute toxicity with SBRT. Toxicity outcomes at 2 years were published recently [13]. Late grade ≥ 2 GU toxicity at 2 years according to RTOG criteria was similar between the treatment arms (2% vs. 3%); however, when studying CTCAE-documented toxicity, patients treated with SBRT had a higher incidence of grade ≥ 2 GU toxicity (+5.7%; p < 0.01). Similarly, cumulative GU toxicity according to RTOG and CTCAE was higher in the SBRT arm. Late GI grade ≥ 2 toxicity was comparable between the treatment groups.
The optimal treatment technique, image guidance, and dose prescription for SBRT in prostate cancer are unknown. While SBRT may be delivered using standard linear accelerators with CT-based image guidance, there are some data to suggest that treatment techniques with intrafractional image guidance and advanced motion management may improve outcome. A subgroup analysis from the PACE‑B trial according to treatment technique in the SBRT arm demonstrated significantly lower rates of worst RTOG grade ≥ 2 acute GU toxicity for patients treated with robotic SBRT as compared to conventional linear accelerators, while GI toxicity was similar [12]. Subgroup analysis at 2 years showed significantly lower rates of late grade ≥ 2 GU and GI toxicity with robotic SBRT compared to C‑arm-based SBRT; however, this was a non-randomized comparison and confounding factors may have influenced the results [13]. Nevertheless, these results regarding reduced GU toxicity may be attributable to a reduced dose to the bladder neck because of the use of multiple non-coplanar beam angles with robotic SBRT. Another explanation would be the use of reduced PTV margins; however, GI toxicity was similar in the PACE‑B trial.
In the past years, MR-guided radiotherapy has been introduced into clinical use, which offers the benefit of continuous intrafractional image guidance and the possibility of gated dose delivery [14]. Recently, a planned interim analysis from the randomized controlled MIRAGE trial was presented at the American Society of Clinical Oncology (ASCO) GU meeting 2022 [15]. All patients received SBRT with 40 Gy in 5 fractions but were randomized either to standard treatment with CT-based image guidance or to MR-guided radiotherapy. Of note, a PTV margin of 2 mm was used with MR-guided radiotherapy, while 4 mm was applied for CT-guided radiotherapy. Acute grade ≥ 2 GU and GI toxicity was significantly reduced with MR-guided radiotherapy. No results regarding late toxicity or efficacy are available as of now. These data suggest that advanced treatment techniques may result in improvements regarding acute and late toxicity. Further research is needed and prospective trials regarding MR-guided radiotherapy in prostate cancer are ongoing [16].
In prostate cancer, a clear relationship between radiotherapy dose and biochemical control has been demonstrated. This has also been established for SBRT [10]. Our trial used a dose of 35 Gy in 5 fractions, which is at the lower end of the dose spectrum for prostate SBRT. Due to differences in dose homogeneity inside the prostate between C‑arm-based and robotic SBRT, a direct comparison between treatment schedules and trial results is somewhat limited. Our study showed a median PSA of 1.18 ng/ml at the last follow-up at 12–15 months, which is comparable to multi-institutional analysis of PSA kinetics after SBRT [17]. Since the PSA nadir was reached 40 months after radiotherapy in this analysis, no definitive conclusions can be drawn from our data at this point. Nevertheless, prospective data from a cohort of 230 patients with low-risk prostate cancer showed a comparable outcome with 35 Gy compared to 36.25 Gy in 5 fractions at 10 years [18].
The preplanned subgroup analysis of treatment frequency demonstrated a higher incidence of acute GI toxicity and late GU toxicity in patients who had daily treatment. This analysis should be regarded as hypothesis generating, as treatment patterns were mostly site associated. In the PACE‑B trial, individual documentation as well as thresholds for prescribing α‑antagonists triggering GU adverse event severity have been shown to considerably differ between physicians and centers [13], which may partly explain our findings. This also concerns the isolated finding of increased nocturia in the every-other-day group, which is in contrast to the increased toxicity seen for other GU and GI toxicity items. Nevertheless, data from the randomized phase II PATRIOT trial showed that a more protracted schedule with weekly treatments as compared to every-other-day treatment significantly reduced acute GI and GU toxicity, highlighting the role of overall treatment time [19, 20].
Limitations of this report are the short overall follow-up and the associated lack of definitive oncological outcome data. Nevertheless, long-term data for 35 Gy in 5 fractions have been published previously. Due to the limited inclusion criteria, we cannot make any statement regarding the use of robotic SBRT for patients with high-risk prostate cancer. The optimal combination of SBRT and ADT remains to be established. Toxicity assessment according to RTOG criteria seems to have a limited sensitivity for detecting moderate GU toxicity, as suggested by the recently reported results of the PACE‑B trial [13]. The results of the subgroup analysis should be interpreted with caution. Furthermore, regarding the analysis of acute toxicities according to CTCAE, the absence of a toxicity was not explicitly reported. The strengths of this analysis include the rigorous quality assurance and collection of patient-reported outcomes and quality of life data.
In summary, our results demonstrate short-term feasibility and tolerability of robotic SBRT for patients with localized prostate cancer. Further research and longer follow-up are necessary to validate this dose regimen and to analyze the role of treatment technique and dose prescription as well as escalation in prostate SBRT.
References
Hamdy FC, Donovan JL, Lane JA et al (2016) 10-year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med. https://doi.org/10.1056/nejmoa1606220
Tree AC, Alexander EJ, As NJV et al (2013) Biological dose escalation and hypofractionation: what is there to be gained and how Will it best be done? Clin Oncol 25:483–498. https://doi.org/10.1016/j.clon.2013.05.003
Höcht S, Aebersold DM, Albrecht C et al (2016) Hypofractionated radiotherapy for localized prostate cancer. Strahlenther Onkol. https://doi.org/10.1007/s00066-016-1041-5
Shelan M, Aebersold DM, Albrecht C et al (2021) Moderately hypofractionated radiotherapy as definitive treatment for localized prostate cancer: Pattern of practice in German-speaking countries. Strahlenther Onkol 197:993–1000. https://doi.org/10.1007/s00066-021-01820-2
Guckenberger M, Baus WW, Blanck O et al (2020) Definition and quality requirements for stereotactic radiotherapy: consensus statement from the DEGRO/DGMP Working Group Stereotactic Radiotherapy and Radiosurgery. Strahlenther Onkol 196:417–420. https://doi.org/10.1007/s00066-020-01603-1
Schmitt D, Blanck O, Gauer T et al (2020) Technological quality requirements for stereotactic radiotherapy. Strahlenther Onkol 196:421–443. https://doi.org/10.1007/s00066-020-01583-2
King CR, Brooks JD, Gill H et al (2009) Stereotactic body radiotherapy for localized prostate cancer: interim results of a prospective phase II clinical trial. Int J Radiat Oncol Biol Phys 73:1043–1048. https://doi.org/10.1016/j.ijrobp.2008.05.059
Jiang P, Krockenberger K, Vonthein R et al (2017) Hypo-fractionated SBRT for localized prostate cancer: a German bi-center single treatment group feasibility trial. Radiat Oncol 12:138. https://doi.org/10.1186/s13014-017-0872-2
Wiegel T, Albers P, Bartkowiak D et al (2021) Results of a randomized trial of treatment modalities in patients with low or early-intermediate risk prostate cancer (PREFERE trial). J Cancer Res Clin 147:235–242. https://doi.org/10.1007/s00432-020-03327-2
Jackson WC, Silva J, Hartman HE et al (2019) Stereotactic body radiation therapy for localized prostate cancer: a systematic review and meta-analysis of over 6,000 patients treated on prospective studies. Int J Radiat Oncol Biology Phys 104:778–789. https://doi.org/10.1016/j.ijrobp.2019.03.051
Widmark A, Gunnlaugsson A, Beckman L et al (2019) Ultra-hypofractionated versus conventionally fractionated radiotherapy for prostate cancer: 5‑year outcomes of the HYPO-RT-PC randomised, non-inferiority, phase 3 trial. Lancet 394:385–395. https://doi.org/10.1016/s0140-6736(19)31131-6
Brand DH, Tree AC, Ostler P et al (2019) Intensity-modulated fractionated radiotherapy versus stereotactic body radiotherapy for prostate cancer (PACE-B): acute toxicity findings from an international, randomised, open-label, phase 3, non-inferiority trial. Lancet Oncol. https://doi.org/10.1016/s1470-2045(19)30569-8
Tree AC, Ostler P, van der Voet H et al (2022) Intensity-modulated radiotherapy versus stereotactic body radiotherapy for prostate cancer (PACE-B): 2‑year toxicity results from an open-label, randomised, phase 3, non-inferiority trial. Lancet Oncol 23:1308–1320. https://doi.org/10.1016/s1470-2045(22)00517-4
Pathmanathan AU, van As NJ, Kerkmeijer LGW et al (2018) Magnetic resonance imaging-guided adaptive radiation therapy: a “game changer” for prostate treatment? Int J Radiat Oncol Biol Phys 100:361–373. https://doi.org/10.1016/j.ijrobp.2017.10.020
Kishan AU, Lamb J, Casado M et al (2022) Magnetic resonance imaging-guided versus computed tomography-guided stereotactic body radiotherapy for prostate cancer (MIRAGE): Interim analysis of a phase III randomized trial. J Clin Oncol 40:255–255. https://doi.org/10.1200/jco.2022.40.6_suppl.255
Ristau J, Hörner-Rieber J, Buchele C et al (2022) Stereotactic MRI-guided radiation therapy for localized prostate cancer (SMILE): a prospective, multicentric phase-II-trial. Radiat Oncol 17:75. https://doi.org/10.1186/s13014-022-02047-w
Jiang NY, Dang AT, Yuan Y et al (2019) Multi-institutional analysis of prostate-specific antigen kinetics after Stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys 105:628–636. https://doi.org/10.1016/j.ijrobp.2019.06.2539
Katz A (2017) Stereotactic body radiotherapy for low-risk prostate cancer: a ten-year analysis. Cureus. https://doi.org/10.7759/cureus.1668
Quon HC, Ong A, Cheung P et al (2018) Once-weekly versus every-other-day stereotactic body radiotherapy in patients with prostate cancer (PATRIOT): A phase 2 randomized trial. Radiother Oncol 127:206–212. https://doi.org/10.1016/j.radonc.2018.02.029
Alayed Y, Quon H, Ong A et al (2020) Accelerating prostate stereotactic ablative body radiotherapy: efficacy and toxicity of a randomized phase II study of 11 versus 29 days overall treatment time (PATRIOT). Radiother Oncol 149:8–13. https://doi.org/10.1016/j.radonc.2020.04.039
Funding
Funding for the trial was provided by the Dr. Rurainski Stiftung (Ettlingen, Germany) and the Saphir Medical Engineering Group (Güstrow, Germany).
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
D. Krug has received honoraria from MSD Sharp & Dohme and Pfizer as well as research funding from Merck KGaA, outside the submitted work. A. Muacevic and C. Fürweger previously received speaker fees from Accuray. O. Blanck is the section editor for medical physics of the journal Strahlentherapie und Onkologie. D. Imhoff, A. Haidenberger, N. Heßler, J. Schäfer, S. Huttenlocher, G. Chatzikonstantinou, U. Ramm, R. König, F. Chun, M. Staehler, C. Rödel, R. Vonthein, and J. Dunst declare that they have no competing interests.
Ethical standards
The study was approved by the ethics committee of the University of Lübeck (leading ethics committee, file number 13–052) as well as the local ethics committees at the participating sites. The trial was also approved by the federal regulatory authority, the Bundesamt für Strahlenschutz (reference number Z5–22463/2–2013-031). The study is monitored by ZKS Lübeck, Germany (protocol number ZKS-121-003). The trial was accredited by the Working Group Radiation Oncology of the German Cancer Society (trial-ID ARO 2013-06). All patients provided written informed consent before enrolment in the clinical trial.
Supplementary Information
66_2023_2044_MOESM2_ESM.docx
Supplementary Table 2a: Results of PORPUS total score—comparison to visit 0 (baseline). Supplementary Table 2b: Results of PORPUS for each domain and total presented as mean and standard deviation.
66_2023_2044_MOESM3_ESM.docx
Supplementary Table 3a: International Prostate Symptom Score (IPSS). Supplementary Table 3b: International Prostate Symptom Score (IPSS)—comparison to screening.
66_2023_2044_MOESM4_ESM.png
Supplementary Fig. 1: PSA kinetics during the study in the full analysis set. Each line represents one individual patient.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Krug, D., Imhoff, D., Haidenberger, A. et al. Robotic stereotactic body radiotherapy for localized prostate cancer: final analysis of the German HYPOSTAT trial. Strahlenther Onkol 199, 565–573 (2023). https://doi.org/10.1007/s00066-023-02044-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00066-023-02044-2